Research
In our group, we address questions of biological and medical relevance using physical methods. Our main tools are various species of scanning probe microscopes. Seeking to descend deeper and deeper into the nanoworld, we place the development of new instruments and methods with high-speed and low-noise characteristics at the foundation of our research. Our applications include visualizing the dynamic interactions of single biomolecules, revealing morphological and mechanical properties of living cells and tissues, exposing biomaterials on the molecular scale, and illuminating transport processes in artificial and natural membranes.
Topics
Nano-Bio-Physics; medical physics; nanomedicine; cell mechanics; nano-analytics; soft matter; surfaces; scanning probe microscopy; force spectroscopy; mechanics and dynamics of single molecules and nanostructures.Atomic Force Microscopy (AFM)
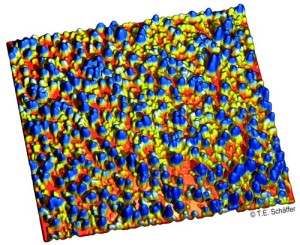
High-resolution atomic force microscope image
of the inner membrane of an oocyte.
Image size: 2.0 µm x 2.0 µm
One of our group’s goals is to image dynamic interactions between single biomolecules in real-time with the atomic force microscope. For example, we have visualized individual binding processes of the tumor suppressor protein p53 with DNA, thereby testing various interaction models.
A further goal is to deduce properties of cellular, sub-cellular and molecular structures from the measurement of their mechanical material properties using force spectroscopy.
In order to better address those questions, we are continuously improving scanning probe microscopy instrumentation and methods. In particular, we are improving resolution and measurement speed by designing and building atomic force microscopes for small cantilevers. These small cantilevers have mechanical resonance frequencies in the Megahertz range.
The increased resolution that we obtain using small cantilevers goes hand-in-hand with increased requirements on the detection system of the atomic force microscope. We therefore develop detectors that sense decreasingly small signals from the cantilever. Using a novel array detector that dynamically adapts to every measurement condition in an optimal way, we were able to increase the AFM detection sensitivity by a factor of five. Also, we have developed a theory for the characterization and optimization of the detection sensitivity.
Scanning Ion Conductance Microscopy (SICM)
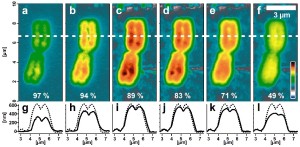
Series of AFM topography images of a re-hydrated human
metaphase chromosome using varying imaging parameters.
Image size: 4.6 µm x 10 µm. Color scale range: 1 µm.
Scanning ion conductance microscopy (SICM) is based on an electrolyte-filled nanopipette. This nanopipette acts as a probe for the locally resolved measurement of ion current over a sample surface submerged in an electrolyte and allows a direct or indirect quantification of various physical quantities such as sample topography, ion concentration, or modulus of elasticity. SICM is a non-contact technique, which is especially useful for the study of soft and fragile samples such as living cells, which are difficult to image with AFM.
We have been using SICM for the contact-free imaging of living cells and of lipid bilayers that are freely suspended over micrometer-sized pores. This allows performing long-term studies of the dynamics of membrane formation and destruction. We have also evaluated and quantified the maximum resolution that can be achieved with SICM. Furthermore, with a recently developed method it becomes possible to measure local mechanical properties of soft samples in a contact-free fashion.
Funding
- DFG-Sonderforschungsbereich Transregio 240 "Platelets"
- DFG-Graduiertenkolleg 2381 "cGMP: From Bedside to Bench"
- DFG-Graduiertenkolleg 2543 "Intraoperative Multisensory Tissue Differentiation in Oncology"